The electron configuration of an atom is a way of describing the arrangement of electrons in the atom's orbitals. It's a fundamental concept in chemistry and physics, and it's crucial for understanding the properties and behavior of atoms and molecules. In this article, we'll focus on the electron configuration of lithium, which is a key element in many chemical reactions and industrial processes.
Introduction to Electron Configurations

Before we dive into the electron configuration of lithium, let’s start with the basics. Electron configurations are typically written in a shorthand notation, using numbers and letters to describe the energy levels and orbitals of an atom. The notation is based on the Aufbau principle, which states that electrons occupy the lowest available energy levels. The electron configuration of an atom is written in the following format: 1s² 2s² 2p⁶, where the numbers represent the energy level, and the letters represent the type of orbital (s, p, d, or f).
Key Points
- The electron configuration of an atom describes the arrangement of electrons in the atom's orbitals.
- The Aufbau principle states that electrons occupy the lowest available energy levels.
- The electron configuration of lithium is 1s² 2s¹.
- Lithium has a single electron in its outermost energy level, which makes it highly reactive.
- The electron configuration of lithium is crucial for understanding its properties and behavior in chemical reactions.
Lithium Electron Configuration
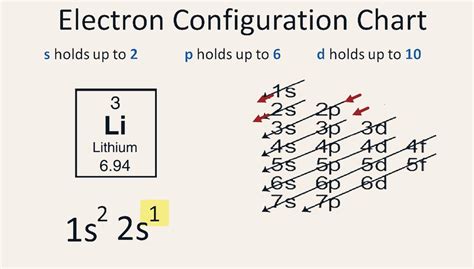
Lithium is an alkali metal with an atomic number of 3, which means it has three electrons. The electron configuration of lithium is 1s² 2s¹, which indicates that the first energy level (1s) is fully occupied with two electrons, and the second energy level (2s) has one electron. This configuration is unique to lithium and is responsible for its chemical properties and behavior.
Understanding the 1s and 2s Orbitals
The 1s orbital is the lowest energy level in an atom, and it can hold a maximum of two electrons. In lithium, the 1s orbital is fully occupied with two electrons, which are paired in a spin-opposed manner. The 2s orbital is the next available energy level, and it can also hold a maximum of two electrons. In lithium, the 2s orbital has one electron, which is unpaired and highly reactive.
| Energy Level | Orbital | Electron Capacity | Electron Occupancy (Lithium) |
|---|---|---|---|
| 1 | 1s | 2 | 2 |
| 2 | 2s | 2 | 1 |

Chemical Properties of Lithium
The electron configuration of lithium is responsible for its chemical properties and behavior. Lithium is a highly reactive metal that readily loses its single electron in the 2s orbital to form a positive ion (Li⁺). This reactivity makes lithium useful in a wide range of applications, including batteries, electronics, and pharmaceuticals.
Lithium-Ion Batteries
Lithium-ion batteries are a type of rechargeable battery that uses lithium ions to store energy. The batteries consist of a positive electrode (cathode) and a negative electrode (anode), separated by an electrolyte. When the battery is charged, lithium ions move from the cathode to the anode, releasing electrons that flow through the external circuit. When the battery is discharged, the lithium ions move back to the cathode, releasing energy that powers the device.
The electron configuration of lithium is crucial for understanding the behavior of lithium-ion batteries. The single electron in the 2s orbital makes lithium highly reactive, which allows it to readily lose and gain electrons during the charging and discharging cycles.
What is the electron configuration of lithium?
+The electron configuration of lithium is 1s² 2s¹, which indicates that the first energy level (1s) is fully occupied with two electrons, and the second energy level (2s) has one electron.
Why is lithium highly reactive?
+Lithium is highly reactive because it has a single electron in its outermost energy level (2s), which makes it readily lose and gain electrons during chemical reactions.
What are some common applications of lithium?
+Lithium is used in a wide range of applications, including batteries, electronics, pharmaceuticals, and nuclear reactions. Its high reactivity and unique electron configuration make it a versatile and valuable element in many industries.
In conclusion, the electron configuration of lithium is a fundamental concept that underlies its chemical properties and behavior. The single electron in the 2s orbital makes lithium highly reactive, which is why it’s often used in batteries and other industrial applications. Understanding the electron configuration of lithium is crucial for appreciating its role in chemistry and physics, and for developing new technologies that utilize its unique properties.