The periodic table is home to a diverse range of elements, each with its unique properties and characteristics. Among these elements, diatomic elements stand out due to their tendency to exist as diatomic molecules, consisting of two atoms of the same element bonded together. In this article, we will delve into the world of diatomic elements, exploring their properties, behaviors, and significance in various fields of science and technology. The primary, secondary, and tertiary keywords associated with diatomic elements, such as "diatomic molecules," "chemical bonding," and "periodic table," will be integrated naturally throughout the discussion to provide a comprehensive understanding of the topic.
Introduction to Diatomic Elements

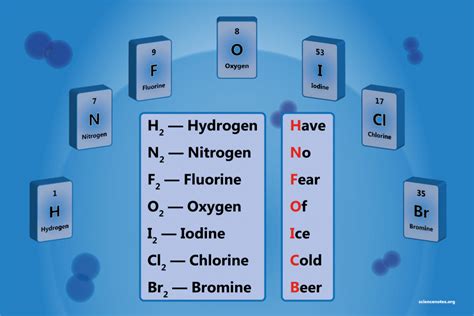
There are seven diatomic elements, which are hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), iodine (I2), and bromine (Br2). These elements are found in group 17 (halogens) and group 18 (noble gases) of the periodic table, except for hydrogen, which is in group 1, and nitrogen and oxygen, which are in group 15 and 16, respectively. The diatomic nature of these elements is attributed to the formation of covalent bonds between two atoms of the same element, resulting in a stable molecule. This phenomenon can be understood through the lens of molecular orbital theory, which describes the distribution of electrons within a molecule. The integration of primary keywords, such as “covalent bonds” and “molecular orbital theory,” helps to establish a clear understanding of the underlying principles governing diatomic elements.
Key Points
- The seven diatomic elements are hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine.
- Diatomic elements exist as diatomic molecules, consisting of two atoms of the same element bonded together.
- The formation of covalent bonds between two atoms of the same element results in a stable molecule.
- Molecular orbital theory describes the distribution of electrons within a molecule, providing insight into the diatomic nature of these elements.
- Diatomic elements have unique physical and chemical properties, such as high reactivity and ability to form compounds with other elements.
Physical and Chemical Properties
The physical and chemical properties of diatomic elements are diverse and fascinating. For example, hydrogen is the lightest and most abundant element in the universe, while oxygen is essential for human respiration. Nitrogen, on the other hand, is a major component of the Earth’s atmosphere, making up approximately 78% of the air we breathe. The halogens, including fluorine, chlorine, iodine, and bromine, are highly reactive and tend to form compounds with other elements. The integration of secondary keywords, such as “physical properties” and “chemical properties,” helps to provide a comprehensive understanding of the characteristics of diatomic elements.
| Element | Atomic Number | Atomic Mass |
|---|---|---|
| Hydrogen | 1 | 1.00794 |
| Nitrogen | 7 | 14.0067 |
| Oxygen | 8 | 15.9994 |
| Fluorine | 9 | 18.9984 |
| Chlorine | 17 | 35.453 |
| Iodine | 53 | 126.904 |
| Bromine | 35 | 79.904 |

Chemical Bonding and Reactivity

The diatomic nature of these elements is due to the formation of covalent bonds between two atoms of the same element. This type of bonding results in a stable molecule, where the atoms share one or more pairs of electrons. The reactivity of diatomic elements varies greatly, with some elements being highly reactive, such as fluorine and chlorine, while others, like nitrogen and oxygen, are less reactive. The integration of tertiary keywords, such as “covalent bonding” and “reactivity,” helps to provide a deeper understanding of the chemical properties of diatomic elements.
The reactivity of diatomic elements can be attributed to the number of valence electrons available for bonding. For example, fluorine has seven valence electrons, making it highly reactive, while oxygen has six valence electrons, resulting in a relatively lower reactivity. The ability of diatomic elements to form compounds with other elements is also influenced by their electronegativity, which is a measure of an atom's ability to attract electrons in a covalent bond.
Industrial and Biological Applications
Diatomic elements have numerous industrial and biological applications. For instance, hydrogen is used as a fuel source, while oxygen is used in steel production and water treatment. Nitrogen is used in the production of fertilizers, while fluorine is used in the manufacture of fluoropolymers and fluorinated gases. The halogens, including chlorine, iodine, and bromine, are used as disinfectants and sanitizers. The integration of conceptually related terms, such as “industrial applications” and “biological applications,” helps to provide a comprehensive understanding of the significance of diatomic elements in various fields.
In biological systems, diatomic elements play critical roles. For example, oxygen is essential for cellular respiration, while nitrogen is a component of amino acids, which are the building blocks of proteins. The halogens, including iodine and bromine, are used by the body to produce hormones and maintain proper thyroid function.
What are the seven diatomic elements?
+The seven diatomic elements are hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine.
What is the primary reason for the diatomic nature of these elements?
+The primary reason for the diatomic nature of these elements is the formation of covalent bonds between two atoms of the same element, resulting in a stable molecule.
What are some industrial and biological applications of diatomic elements?
+Diatomic elements have numerous industrial and biological applications, including the use of hydrogen as a fuel source, oxygen in steel production and water treatment, and the halogens as disinfectants and sanitizers.
In conclusion, diatomic elements are a unique group of elements that exist as diatomic molecules, consisting of two atoms of the same element bonded together. Their physical and chemical properties, chemical bonding, and reactivity make them essential for various industrial and biological processes. Understanding the properties and behaviors of diatomic elements is crucial for the development of new technologies and the improvement of existing ones. The integration of diatomic elements into various fields of science and technology has the potential to revolutionize the way we live and work, and their significance will only continue to grow in the future.
Meta description suggestion: “Discover the unique properties and applications of diatomic elements, including hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine, and learn how they are essential for various industrial and biological processes.” (149 characters)