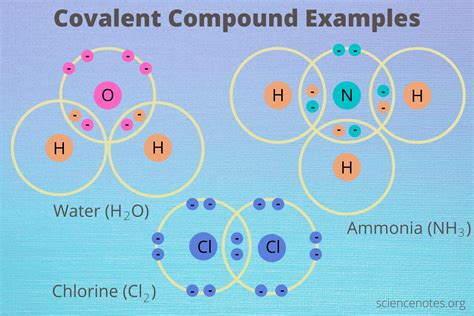
Covalent compounds are a class of chemical substances that are formed when two or more atoms share one or more pairs of electrons to achieve a stable electronic configuration. This type of chemical bonding is characteristic of molecules, which are the basic units of covalent compounds. The formation of covalent bonds involves the overlap of atomic orbitals, resulting in a shared pair of electrons that are attracted to the nuclei of the atoms involved. Covalent compounds can be found in a wide range of substances, from simple molecules like water and carbon dioxide to complex biomolecules such as proteins and nucleic acids.
Characteristics and Examples of Covalent Compounds

Covalent compounds exhibit several distinct characteristics, including low melting and boiling points compared to ionic compounds, solubility in non-polar solvents, and the ability to conduct electricity when dissolved in water or melted. One of the primary advantages of covalent compounds is their versatility, as they can be found in a vast array of substances with diverse properties and applications. For instance, methane (CH4) is a simple covalent compound that serves as the main component of natural gas, while silicon dioxide (SiO2) is a covalent compound that is commonly found in sand and quartz.
Types of Covalent Compounds
Covalent compounds can be broadly classified into several categories, including polar covalent compounds, non-polar covalent compounds, and metallic covalent compounds. Polar covalent compounds are characterized by a significant difference in electronegativity between the atoms involved in the covalent bond, resulting in a partial positive charge on one atom and a partial negative charge on the other. Examples of polar covalent compounds include hydrogen fluoride (HF) and ammonia (NH3). In contrast, non-polar covalent compounds have a relatively small difference in electronegativity between the atoms involved, resulting in a more symmetrical distribution of electrons. Examples of non-polar covalent compounds include oxygen (O2) and nitrogen (N2).
| Type of Covalent Compound | Examples |
|---|---|
| Polar Covalent Compounds | Hydrogen Fluoride (HF), Ammonia (NH3), Water (H2O) |
| Non-Polar Covalent Compounds | Oxygen (O2), Nitrogen (N2), Carbon Dioxide (CO2) |
| Metallic Covalent Compounds | Silicon Dioxide (SiO2), Germanium (Ge), Graphite (C) |

Key Points
- Covalent compounds are formed through the sharing of electron pairs between atoms.
- Polar covalent compounds have a significant difference in electronegativity between atoms, resulting in partial charges.
- Non-polar covalent compounds have a relatively small difference in electronegativity, resulting in a symmetrical distribution of electrons.
- Metallic covalent compounds exhibit properties of both metals and covalent compounds, such as high melting points and conductivity.
- The polarity of covalent compounds is crucial for predicting their physical and chemical properties.
Covalent compounds play a vital role in various fields, including chemistry, biology, and materials science. Understanding the properties and characteristics of covalent compounds is essential for developing new materials, designing chemical reactions, and predicting the behavior of molecules in different environments. By recognizing the different types of covalent compounds and their unique properties, researchers and scientists can unlock new applications and innovations in a wide range of fields.
What is the main difference between polar and non-polar covalent compounds?
+The main difference between polar and non-polar covalent compounds is the distribution of electrons in the covalent bond. Polar covalent compounds have a significant difference in electronegativity between the atoms involved, resulting in partial charges, while non-polar covalent compounds have a relatively small difference in electronegativity, resulting in a symmetrical distribution of electrons.
What are some common examples of covalent compounds?
+Some common examples of covalent compounds include water (H2O), carbon dioxide (CO2), methane (CH4), ammonia (NH3), and silicon dioxide (SiO2).
What is the importance of covalent compounds in biology?
+Covalent compounds play a crucial role in biology, as they are the building blocks of biomolecules such as proteins, nucleic acids, and carbohydrates. Understanding the properties and characteristics of covalent compounds is essential for understanding the structure and function of biomolecules and their interactions in living organisms.
Meta Description: Learn about covalent compounds, including their characteristics, types, and examples, and discover their importance in chemistry, biology, and materials science.