Hydrosulfuric acid, commonly referred to as hydrogen sulfide, is a chemical compound with the formula H₂S. This compound is a colorless, flammable, and extremely hazardous gas with a characteristic odor of rotten eggs. The chemical formula H₂S indicates that one molecule of hydrosulfuric acid consists of two hydrogen atoms bonded to a single sulfur atom. Understanding the composition and properties of hydrosulfuric acid is crucial due to its wide range of applications and its potential as an environmental and health hazard.
Key Points
- The chemical formula for hydrosulfuric acid is H₂S, indicating two hydrogen atoms and one sulfur atom per molecule.
- Hydrosulfuric acid is highly toxic and can be lethal in high concentrations, affecting the nervous system and causing respiratory failure.
- It is used in various industrial processes, including the production of sulfur, sulfuric acid, and as a precursor for the synthesis of other chemicals.
- Natural sources of hydrosulfuric acid include volcanic activity, natural gas, and the decomposition of organic matter, particularly in environments with low oxygen levels.
- The detection and measurement of H₂S are critical in occupational safety, environmental monitoring, and public health due to its hazardous nature.
Chemical Properties and Reactions
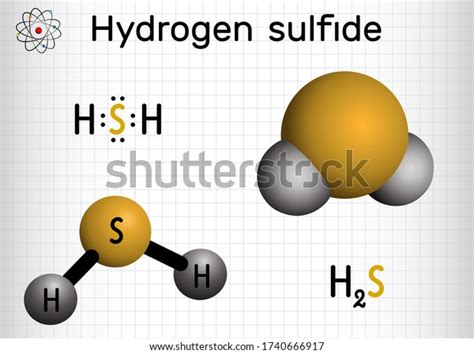
Hydrosulfuric acid exhibits several notable chemical properties. It is a weak acid, meaning it does not fully dissociate in water, resulting in a relatively low concentration of hydrogen ions. The acid dissociates according to the equation H₂S ⇌ HS⁻ + H⁺, with the HS⁻ ion further dissociating to S²⁻ and H⁺ in a second step, though this occurs to a much lesser extent. Hydrosulfuric acid reacts with metals to form metal sulfides and with metal oxides to form sulfites or sulfates, depending on the metal and the conditions of the reaction.
Industrial Applications and Environmental Impact
The industrial significance of hydrosulfuric acid lies in its use as a precursor to sulfur and sulfuric acid, which are vital components in the manufacture of fertilizers, pesticides, and other chemicals. However, the production and handling of H₂S pose significant environmental and health risks due to its toxicity. The release of hydrosulfuric acid into the atmosphere contributes to air pollution, and its presence in water can lead to the formation of acidic and oxygen-depleted environments, harming aquatic life.
| Property | Value |
|---|---|
| Molecular Weight | 34.08 g/mol |
| Boiling Point | -60.3°C |
| Melting Point | -85.5°C |
| Density | 1.363 g/L |

Safety and Health Considerations

Exposure to hydrosulfuric acid can occur through inhalation, ingestion, or skin contact, with the severity of the effects depending on the concentration and duration of exposure. At low concentrations, H₂S can cause irritation to the eyes, nose, and throat, while higher concentrations can lead to more severe health effects, including headaches, dizziness, and even death due to respiratory failure. The safety protocols for handling hydrosulfuric acid include the use of protective clothing, respirators, and working in well-ventilated areas to minimize exposure risks.
Detection and Measurement Techniques
The detection and measurement of hydrosulfuric acid are crucial for occupational safety and environmental monitoring. Several methods are available, including colorimetric tests, gas detectors, and chromatographic techniques. These methods vary in sensitivity, selectivity, and ease of use, with the choice of method depending on the specific application and the concentration range of interest.
What is the primary use of hydrosulfuric acid in industry?
+Hydrosulfuric acid is primarily used as a precursor for the production of sulfur and sulfuric acid, which are essential in the manufacture of fertilizers, pesticides, and other chemicals.
What are the health risks associated with exposure to hydrosulfuric acid?
+Exposure to hydrosulfuric acid can cause a range of health effects, from mild irritation of the eyes, nose, and throat at low concentrations to severe effects such as headaches, dizziness, and respiratory failure at higher concentrations.
How is hydrosulfuric acid detected and measured in the environment?
+Hydrosulfuric acid can be detected and measured using various methods, including colorimetric tests, gas detectors, and chromatographic techniques, with the choice of method depending on the specific application and the concentration range of interest.
In conclusion, hydrosulfuric acid, with the chemical formula H₂S, is a compound of significant industrial and environmental importance. Its unique chemical properties make it a versatile precursor for various chemical syntheses, but its high toxicity and potential environmental impact necessitate careful handling and strict safety protocols. Understanding the properties, applications, and risks associated with hydrosulfuric acid is essential for its safe and effective use in industrial processes and for mitigating its environmental and health impacts.
